Objective
In this lab students will learn about atomic energy levels, atomic emission spectroscopy, and the spectral “fingerprints” of elements. Students will closely observe the spectrum of light produced by atomic emission gas discharge tubes using simple spectroscopes. They will record the spectra they observe.
Background
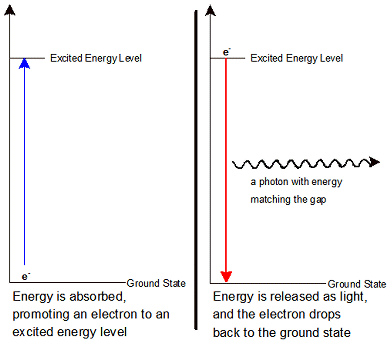
The electrons in an atom occupy different energy levels, as you know. When all of the electrons are at the lowest possible energy level they are said to be in the ground state. Electrons do not always stay in the ground state. Sometimes they can be promoted to a higher-energy electron shell. This can happen in two ways. First, the electron can absorb a photon of just the right amount of energy to move it from one quantum shell to another. Second, when atoms are heated or energized with electricity their electrons can gain energy. This promotes them to the higher-energy shell. When an electron is in a higher-energy shell it is said to be in an excited state.
Electrons in excited states do not usually stay in them for very long. When electrons lose their energy they do so by emitting a photon of light. Photons are particles with energy but no mass. Their energy is directly proportional to the frequency of the light (remember: E = hf). The photons emitted precisely match the quantum energy difference between the excited state and the ground state.
For different elements the spacing between the ground state and the higher energy levels is different. This gives rise to a way to uniquely identify elements based on their spectrum. A spectrum is the scientific name for a rainbow: light broken into the different wavelengths that make it up. You can see spectra using a spectroscope, a prism, or a diffraction grating. A spectroscope is a device which uses a diffraction grating to create a visual spectrum in a way that places the spectrum on a scale. This enables the user to measure the wavelengths of light being observed. The back of an ordinary CD is a reflective diffraction grating. Atoms produce very sharp lines in a spectrum when they are heated. You will look at these lines in this lab. These lines show the energy differences between different excited states and lower states, including the ground state. The atomic spectrum of hydrogen is shown below.

H Emission Spectrum
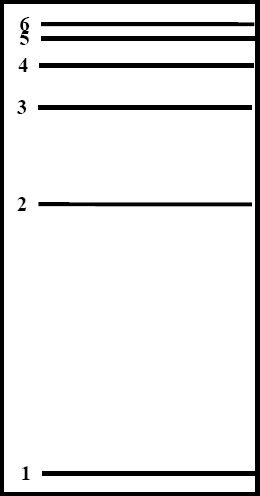
By recording the wavelength of each line it is possible to calculate the energy being emitted by the atoms of hydrogen in the tube. Each different amount of energy represents a different transition from a higher (excited) state to a lower state. For example, the transition of an electron in a hydrogen atom from the third level to the second produces a red line at 657 nm. When an electron moves from the fourth level to the second level in a hydrogen atom a photon with a wavelength of 486 nm is produced. The 434 nm line is produced by a transition from the fifth level to the second and the 410 nm line is produced when an electron moves from the sixth level to the second. A diagram of these energy levels in a hydrogen atom is shown at right.
Because every element has a unique spectrum the spectrum of an element can be used to identify it. Distant stars are too far away for us to take a sample to analyze in a lab. Even so, we can gather information about what they are made of by looking at the spectrum of light they produce. By collecting data here on Earth for every element we can record their spectral “fingerprints”. These can be used to identify them in far off stars and galaxies.
