Lab: Solubility
Objective
Students will learn about the principle that ‘like dissolves like’. To do so they will quantify how much salt can dissolve in two solvents, one polar and one non-polar. Solutions are homogeneous mixtures of two or more components. That is, once they are mixed the solution looks the same throughout. The solvent is whatever substance is present in a larger amount. Water is the most common everyday solvent.
Overview
In this lab students measure 10 mL of distilled water into one 50 mL beaker and into the second beaker, 10 mL of ethyl alcohol. Students then add carefully measured amounts of salt and stir until no more salt is present in solid form or until no more dissolves. In order to find out how much of the added salt was dissolved students pour off the liquid in each beaker, being careful to leave all of the remaining solid behind. Next, students evaporate any remaining liquid using a hot plate on a low setting. Finally, students find the mass of leftover solid and use the measurement to evaluate which solvent is the better one for dissolving salt.
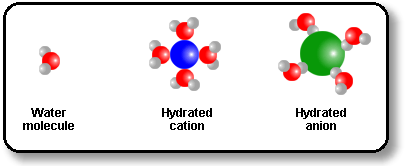
The Chemist’s rule of thumb about solubility is that ‘like dissolves like’. That is, polar solvents dissolve polar and ionic compounds but only non-polar solvents dissolve non-polar substances. Water is good at dissolving salts because salts consist of cations and anions. Water (H2O) has a positive end (near the H atoms) and a negative end (near the O atom). Water can hydrate ions of a salt by surrounding cations with the oxygen atom pointing toward the positively charged particle and by surrounding anions with the hydrogen atoms pointing toward the negatively charged particle. Water is polar because of the large electronegativity difference between oxygen and hydrogen. Nonpolar solvents like ethanol are not as good at hydrating ions. They have more favorable interactions with other nonpolar molecules than with ions and so do not dissolve salts.
A substance will dissolve in water (or some other solvent) when there is an intermolecular attraction between the solvent molecules and the solute molecules. Intermolecular attractions (attractive forces between molecules) can take several forms. When a salt dissolves in water the cations and anions are attracted to the positive and negative poles of the water molecule. This is attraction between charged particles. When a non-polar solute dissolves in a non-polar solvent the intermolecular interactions take a different form. When non-polar molecules can get physically (very) close to each other they experience what are called dispersion forces. These forces cause the molecules to be attracted to each other and stick together. Polar and non-polar molecules do not mix well because the non-polar molecules are not attracted strongly enough to the polar water molecules to make up for pulling the molecules away from each other. In order to dissolve, the solvent molecules have to be pulled away from each other and the solute molecules have to be pulled away from each other. If they are not strongly attracted to each other then they won’t stick to each other with enough force to equal the force needed to separate the molecules in the first place.
Materials
- ~8.0 g salt
- 2 50 mL beakers
- 10 mL graduated cylinder
- 10 mL distilled water
- 10 mL ethyl alcohol
- lab marker
- hot plate
- tongs
- lab balance
- safety glasses!
Safety
- If you choose not to wear safety glasses you are choosing to sit out the lab
- Ethyl alcohol is an eye irritant: wash out eyes immediately if you get any in your eyes
- Ethyl alcohol has some powerful fumes, be careful to breathe them as little as possible
- Ethyl alcohol is flammable: before evaporating, pour as much as possible into the waste beaker
- Hot objects can cause fires and burns: use caution at all times when using the hot plate
Measuring Solubility
Solubility in Water
Water is a very polar molecule. Salt is an ionic solid. Ionic solids are made up of positively charged cations and negatively charged anions. The positive and negative poles of water molecules are able to attract these ions so strongly that they break apart and move freely through the water. But are there limits in the amount of salt that can dissolve in a particular volume of water? Your task is to find out.
Objective 1
Find the maximum amount of salt that will completely dissolve in 10 mL of water. Express your answer in g per L. (1 L = 1,000 mL) For hints about how to proceed, read the Overview section of this lab and pay attention when the teacher introduces it.
- Describe the experiments you will perform:
- What are the things you need to measure in your work in the lab for this objective?
- What are the important things you need to control when making your measurements to be sure to answer the objective?
- What do you think the results of the experiments will be?
- How are you going to decide when you have completed the objective?
Design your own data table for this part of the lab. Be sure to plan carefully and leave room for multiple trials of the same experimental set-up, if needed. Also, remember to leave room to enter average values or other descriptive data. You may find it helpful to use a ruler. Sketch your data table on a separate piece of paper and show it to your teacher for approval before putting the final version here. You must obtain your teacher’s initials before putting your data table here.
Check-in ______
Summary for Objective One
Answer the questions below before moving on to Objective Two.
- What is the maximum amount of salt that will dissolve in in 10 mL of water?
- Calculate the solubility of salt in water in g/L. Do this by expressing your result in units of g/mL and using the conversion factor given at the beginning of this objective.
- Is water a good solvent for salt? (You may re-evaluate your answer after doing experiments with ethyl alcohol). Provide data to support your answer.
Check-in ______
When you have completed this objective check in with your teacher. This is a required part of the lab and your teacher’s initials are required before you can move on to the next objective.
Solubility in Ethyl Alcohol
Ethyl alcohol is a slightly polar molecule. Part of it is polar and part of it is non-polar. Salt is an ionic solid. The positive and negative poles of ethyl alcohol molecules are too weak to be able to attract ions strongly enough that they break apart and move freely through the liquid. Ethyl alcohol is weakly polar but its non-polar part makes it unable to dissolve ionic salts. Or does it? Your task is to find out.
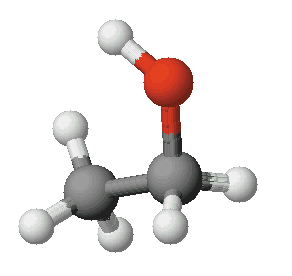 Ethyl Alcohol
Ethyl AlcoholObjective Two
Find the maximum amount of salt that will completely dissolve in 10 mL of ethyl alcohol. Express your answer in g per L. (1 L = 1,000 mL) For hints about how to proceed, read the Overview section of this lab and pay attention when the teacher introduces it.
- Describe the experiments you will perform:
- What are the things you need to measure in your work in the lab for this objective?
- What are the important things you need to control when making your measurements to be sure to answer the objective?
- What do you think the results of the experiments will be?
- How are you going to decide when you have completed the objective?
Design your own data table for this part of the lab. Be sure to plan carefully and leave room for multiple trials of the same experimental set-up, if needed. Also, remember to leave room to enter average values or other descriptive data. You may find it helpful to use a ruler. Sketch your data table on a separate piece of paper and show it to your teacher for approval before putting the final version here. You must obtain your teacher’s initials before putting your data table here.
Check-in ______
Summary for Objective Two
Answer the questions below.
- What is the maximum amount of salt that will dissolve in in 10 mL of ethyl alcohol?
- Calculate the solubility of salt in ethyl alcohol in g/L. Do this by expressing your result in units of g/mL and using the conversion factor given at the beginning of this objective.
- Is ethyl alcohol a good solvent for salt?
- Which is the better solvent for salt: water or ethyl alcohol? Provide data to support your answer.
Check-in ______
When you have completed this objective check in with your teacher. This is a required part of the lab and your teacher’s initials are required before you can move on to the next objective.
Lab Report
The report you hand in for this lab will have two parts:
First, you will hand in your procedure with data and the answers to the summary questions, including all check-in initials from your teacher.
Second, you will perform some at-home research to answer some questions raised by this lab. Contrary to popular belief, chemistry is relevant to your everyday life. You will do some experiments at home to find out how. Write up the results of your experiments along with the answers to the questions below. Note: some of the questions require further reading! Some web links are provided but additional research (even just surfing the web for it) will be necessary. Provide a complete bibliography for all sources used. For all at-home experiments use only very small amounts so as not to waste the materials! Use no more than a tablespoon or so at a time.
- Describe the process by which solvents
dissolve solutes. This must be a thoroughly researched answer and will require the
consultation of several sources in order to receive full credit. A guide for your research:
- Find and read several sources; use the index of a text, the library catalog and the Internet (when using the index, read pages near the page referenced; use what you read to help you find new keywords to look up in the index)
- Work to understand the material you read; if you don’t understand it, keep working; find other sources to explain the words and ideas you don’t understand
- Write down what you have learned in your own words; you don’t learn anything if you just copy someone else’s work!
Elmhurst College Virtual ChemBook
http://www.inquiryinaction.org/chemistryreview/dissolving/
http://www.inquiryinaction.org/chemistryreview/solids/
Solutions: http://www.elmhurst.edu/~chm/vchembook/170Asolubility.html
Mixtures: http://www.elmhurst.edu/~chm/vchembook/106Amixture.html
Solubility of Salts: http://www.elmhurst.edu/~chm/vchembook/171solublesalts.html (includes links to two animations: watch them!)
- Is vegetable oil a polar or a nonpolar solvent? Does salt dissolve in oil? Explain why or why not. Devise an experiment to find out and describe it in your report using the methods we have been using in the lab to design experiments. Do the experiment at home and report the results. Note: you will not be able to make the oil evaporate so do not heat up the oil as part of your experiment! Try adding tiny amounts of salt to oil in a clear glass as a starting point.
- Table sugar is the substance chemists call sucrose. Sucrose is an organic chemical of biological origin. It is a covalently bonded compound. As you know, it dissolves very well in water, a polar solvent. Will sugar dissolve in ethyl alcohol? Will sugar dissolve in oil, a non-polar solvent? Make a prediction and explain your answer before you do an experiment. Do an experiment at home and report the results—you can use household isopropyl alcohol (rubbing alcohol) as a substitute for ethyl alcohol.
- Why is it not possible to mix oil and water? Will alcohol and oil mix? Does alcohol dissolve in water? Make a prediction and explain your answer before you do an experiment. Use what you now know about how the polarity of a compound determines what kind of solvent it will dissolve in. Do an experiment at home using rubbing alcohol as a substitute for ethyl alcohol. Try adding a little of the compound you want to dissolve to a larger amount of the compound you are using as the solvent. How could you tell if alcohol dissolved in water? Try adding food coloring to only one or the other.
- Rub a plastic ruler or a comb with a piece of paper. When you do this you create a separation of electric charges: the plastic now has a static electric charge. Hold the charged plastic near a smoothly and slowly flowing stream of water. Describe what happens. (This may not work if the air is too humid). Explain what happens in terms of the polar nature of water molecules: they have positively and negatively charged parts. Remember: opposite charges attract. Draw a picture as part of your answer to this question.