Lab: Stoichiometry
Objective
In this lab students will dissolve a known amount of sodium carbonate Na2CO3 in water and mix it with a solution containing excess calcium chloride (CaCl2). The chemical reaction that occurs is
In the chemical equation above the notation (aq) means ‘aqueous’, that is, dissolved in water. The notation (s) means solid. These notations are included to show that a precipitate forms in the reaction. A precipitate is a solid compound that results from the reaction of two soluble compounds in a solution. Precipitates form due to their low solubility in water. The solid precipitate will be collected, dried and weighed. The mass of calcium carbonate will then be compared with the mass predicted by stoichiometric calculations.
Overview
Stoichiometry is the technique of using the molar ratios in balanced chemical equations to calculate the amount of reactants or products. In this lab a sample of sodium carbonate (Na2CO3) with a mass of 4 - 5 g is dissolved in distilled water. Using distilled water reduces the chance that any excess carbonate ions may be present. Next, a solution of calcium chloride (CaCl2) is mixed with the solution of Na2CO3. The calcium chloride solution has a concentration of 1.0 mol/L (usually written 1.0 M). In this procedure a volume of 60 mL of this solution is used. This volume contains 0.060 L × 1.0 mol/1 L = 0.060 mol CaCl2. This amount of CaCl2 is in stoichiometric excess over the Na2CO3 that will be used. This makes the Na2CO3 the limiting reagent and the amount of calcium carbonate (CaCO3) produced will depend only on how much Na2CO3 is present.
Calcium carbonate has very low solubility in water and is usually considered completely insoluble. This substance is familiarly known as chalk, limestone, or marble. It is also the primary component of the shells of marine mollusks and is a very common source of calcium in calcium supplement pills. In the reaction carried out in this lab the CaCO3 forms by the chemical reaction of sodium carbonate with calcium chloride. The amount that forms is determined by the molar ratio between these two chemicals as seen in the chemical equation given above.
This lab is designed to provide data which demonstrate the validity of the mathematical techniques of stoichiometry. By both calculating the amount of CaCO3 that forms and measuring it in the lab it will be possible to calculate the percent yield for the reaction. The percent yield is a measure of the efficiency of the reaction in producing products. It is calculated by dividing the amount measured in the lab by the amount predicted using stoichiometry and multiplying by 100%.
3.97 g --------- × 100% = 92% 4.32 gFor example, if stoichiometry predicts that the reaction will produce 4.32 g but the lab measurement is 3.97 g then the percent yield is:
Materials
- 60 mL Calcium chloride solution (1.0 M)
- 4 - 5 g Sodium carbonate monohydrate
- Distilled water
- Lab Balance
- 1 250-mL beaker
- 100-mL graduated cylinder
- vacuum filter funnel
- vacuum filter flask
- quantitative filter paper
- vacuum tube
- sink aspirator
- metal tongs
- watch glass
- metal scoop
- forceps
- glass stirring rod
with rubber policeman - wash bottle filled with distilled water
Safety
- If you choose not to wear safety glasses you are choosing to sit out the lab.
- Solid powdered sodium carbonate is an eye and respiratory tract irritant. It is also slightly toxic by ingestion. Avoid contact and wash hands after handling it. If exposed, remove victim to fresh air. If in eyes, rinse with cold water for up to 15 minutes.
- Sodium carbonate is a strong base when dissolved in water and can cause irritation of the skin. Avoid contact and wash hands after handling it. If in eyes, rinse with cold water as soon as possible.
- Calcium chloride solution is slightly toxic by ingestion. Call a physician immediately if ingested.
- The filter flasks are designed to withstand the pressures involved in vacuum filtration. However, whenever glass is subjected to differences in gas pressure eye protection and all due caution are required.
- The oven will be kept at a temperature of about 110°C. This is hot enough to burn so proper use of tongs when handling hot glassware is required.
- Wash hands thoroughly with soap and water before leaving the laboratory.
Stoichiometry Procedure
Precipitation Reaction
In this part of the lab you will make a solution of sodium carbonate and mix it with a solution of calcium chloride. Be careful to observe what happens. Read the following step-by-step procedure in its entirety before carrying it out. Before even collecting your materials, plan out the data you will need to collect and make a data table to put it in. Consider, for example, that you will need to know the mass of containers you use for weighing.
- Using appropriate equipment, measure out an amount of sodium carbonate from between 4 and 5 grams. Do not try to get any exact amount such as exactly 4.00 g or 5.00 g. Instead, simply measure out an amount between these extremes and record its exact mass.
- Measure 100 mL of distilled water and pour it carefully into the 250-mL beaker.
- Carefully add the sodium carbonate to the water using the metal scoop. Be sure no solid remains in the container you used to weigh it.
- Stir the mixture you have made until the sodium carbonate has completely dissolved.
- Measure 60 mL of the 1.0 M calcium chloride solution.
- Using the stirring rod to guide the flow of the solution, pour it into the 250-mL beaker containing the sodium carbonate solution. Observe the formation of a white precipitate as you do so. It will appear as a white cloudiness in the water and is in fact a collection of many, many tiny solid particles of calcium carbonate.
You need to design your own data table to capture the data for this part of the lab. You may find it helpful to use a ruler. Sketch your data table on a separate piece of paper and show it to your teacher for approval before putting the final version here. You must obtain your teacher’s initials before putting your data table here.
Check-in ______
Summary for Precipitation Reaction
Answer the questions below before moving on to Filtration and Drying.
- Use the mass of sodium carbonate to predict the mass of calcium carbonate that will form in your experiment. Note: if the monohydrate is used then the molar mass is not 105.98 g/mol but 124.00 g/mol because of the added mass of one mole of water per mole of sodium carbonate. This water is part of the crystal and will not make the white powder look wet.
- Say you used one gram more of the sodium carbonate than you did. How would that affect the amount of calcium carbonate you calculate will be formed? How would it affect the amount you actually collect?
- If you used water that already had some sodium carbonate dissolved in it how would that affect the mass of calcium carbonate that you actually collect? How would that compare to the amount of calcium carbonate you calculated would result from the reaction? Explain.
- If you dissolve the sodium carbonate in water that already had some calcium chloride in it how would that affect the mass of calcium carbonate that you actually collect? How would that compare to the amount of calcium carbonate you calculated would result from the reaction? Explain.
Check-in ______
When you have completed this objective check in with your teacher. This is a required part of the lab and your teacher’s initials are required before you can move on to the next objective.
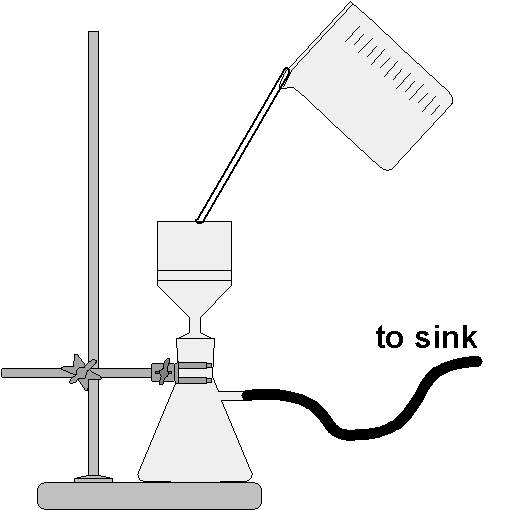
Filtration Procedure
In this part of the lab you will filter the solid calcium carbonate from the water. In the next you will dry the solid material on the filter paper in order to be able to weigh it to find the mass of the precipitate. This will allow you to see whether the mass you measure matches your stoichiometric calculation. Read the following step-by-step procedure in its entirety before carrying it out.
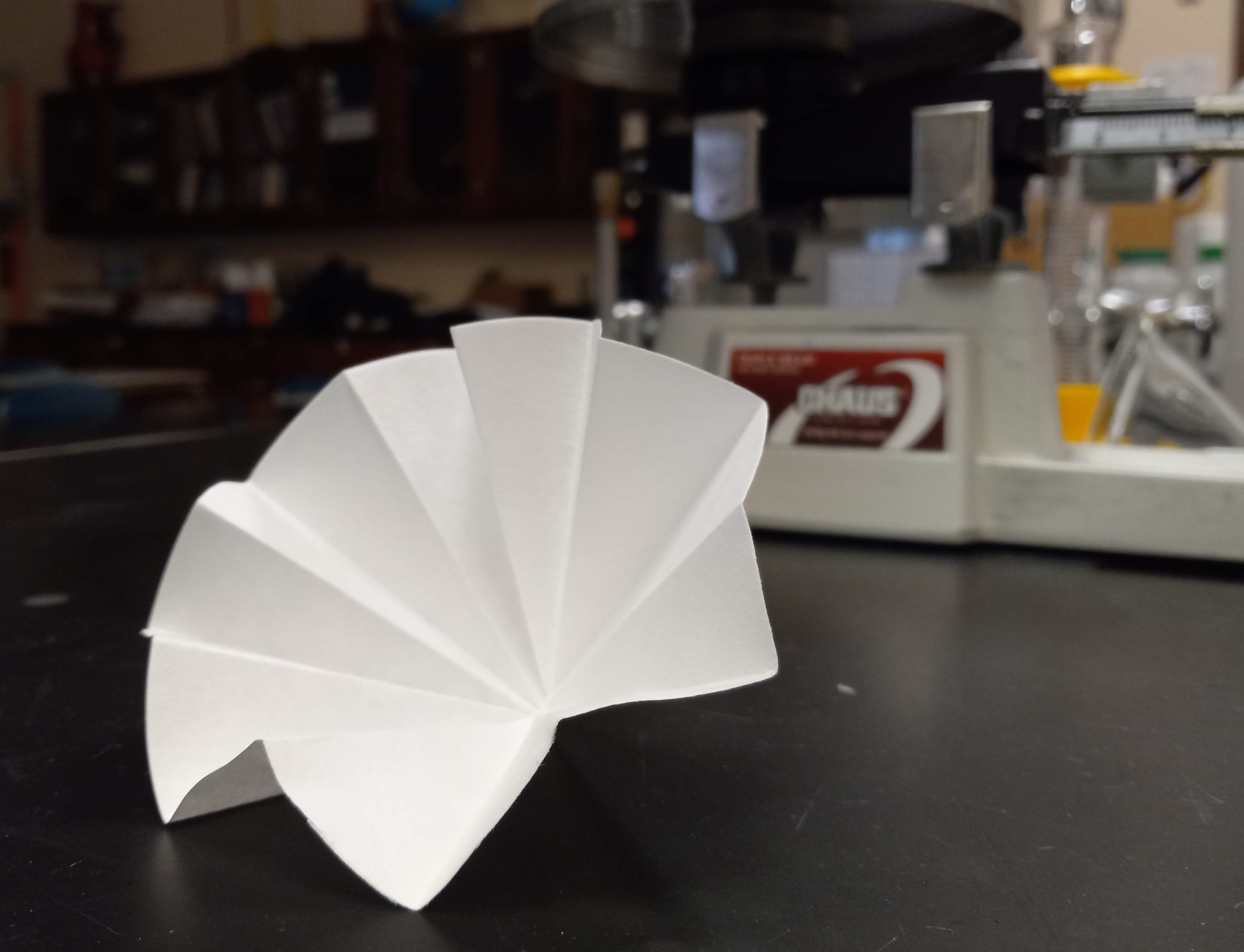 |
| Fluted Filter Paper (step 3) |
 |
| Decanting (step 6) |
- After completing the previous procedure, allow the precipitate to settle to the bottom of the beaker.
- Obtain and find the exact mass of a piece of quantitative filter paper. Record the mass in the data table.
- Fold the filter paper into a fluted shape so that it will fit in the funnel. Make it look like the image at right. Your teacher can show you how to do this.
- Place the folded filter paper into your funnel. Place the funnel in the top of a 250-mL Erlenmeyer flask or onto a funnel support over such a flask.
- Pay attention to the following as you take the next step:
- Do not let that material collect on the filter paper until almost all of the supernatant has been filtered.
- Do not over-fill the funnel! Liquid which overflows can no longer be filtered.
- Finally, do not exceed the capacity of the flask: it can hold a maximum of about 250 mL.
- Use a stirring rod to guide the flow of liquid as you pour it into the filter. The technical language used for this is to decant the supernatant (the liquid above the settled precipitate) into the funnel. (To decant means to pour in such a way as to leave the solid material on the bottom of the beaker.)
- When all but about 10 mL of the liquid has been filtered swirl the beaker to suspend the solid. Transfer this to the funnel so that all of the solid is captured on the filter paper.
- Use the wash bottle to gently wash any remaining solid into the funnel while holding the beaker upright so it drains directly into the funnel while you wash. Your teacher will demonstrate this technique for you.
- After all of the water has drained from the filter, rinse it with water to wash it. Rinse the solid three times with small amounts of distilled water from the wash bottle. This will remove dissolved sodium chloride from the solid calcium carbonate.
Drying Procedure
- Obtain a watch glass and measure its exact mass. Using forceps (tweezers), remove the filter paper from the funnel and place it on the watch glass. Be careful not to tear the filter paper or to lose any of the solid material.
- Place the watch glass with the filter paper on it into the drying oven, which will be set for between 110°C and 120°C.
- Allow the filter paper to dry for 10 - 15 minutes. When this time has passed take the watch glass out of the oven using the metal tongs. Use a metal scoop to gently break up the calcium carbonate into small pieces.
- Place the watch glass back in the oven for 5 more minutes.
- Take out of the oven again with the tongs and set aside to cool. Once cool (in 2 - 3 min), weigh it with the filter paper and calcium carbonate on it. Record this mass in your data table.
- Place the watch glass back in the oven for another five minutes, take out, let cool, and weigh again. Repeat this process until to successive weighings are no more than 0.2 g apart.
- The filter paper can be thrown in the trash. The filtrate (the liquid which has been filtered) can go down the drain. It contains only harmless calcium, sodium, and chloride ions.
- Carefully wash all lab equipment with water containing detergent. If a white film persists on the glass then use a small amount of vinegar to wash it. The acid will make the carbonate soluble and easy to wash away. Do not scrub or scrape the glassware. Rinse completely at least three times before hanging it up to dry.
| Filtration and Drying Data | |
| Mass of Filter Paper (g) | |
| Mass of Watch Glass (g) | |
| Mass of Watch Glass, Filter Paper, and CaCO3 | |
| First Weighing (g) | |
| Second Weighing (g) | |
| Third Weighing (g) | |
| Circle the final mass above | |
| Mass of Dry CaCO3 | |
Collect your data using the data table provided. You must obtain your teacher’s initials before putting away your equipment.
Check-in ______
Summary for Filtration and Drying
Answer the questions below.
- What mass of CaCO3 did you measure in your lab work?
- Is the mass you measured close to the expected mass you calculated based on stoichiometry? Calculate a percent difference between the two. Subtract the calculated value from the measured value and divide the result by the calculated value. Finally, multiply by 100%. If your measured value was smaller, then the percent difference will be negative. If your measure value was bigger, then the percent difference will be positive.
- Would the mass of calcium carbonate that you measure be made larger or smaller if it were not completely dry? Explain.
- Would the mass of calcium carbonate that you measure be made larger or smaller if you did not wash the precipitate before drying it? Explain.
- Using the technique described in the Overview for this lab, calculate the percent yield for your reaction.
- Explain any differences between your measurement and the calculation of the mass of calcium carbonate. In particular, comment on why the percent yield was (likely) not 100%.
Check-in ______
Lab Report
Write a full formal lab report for this lab. Please refer to the handout you received at the beginning of the year titled Lab Report Writing. Use the following questions to guide your writing in the Analysis and Conclusion sections of your report. For the analysis the questions are those you answered during the lab in the lab handout.
Analysis:- Use the mass of sodium carbonate to predict the mass of calcium carbonate that will form in your experiment. Note: if the monohydrate is used then the molar mass is not 105.98 g/mol but 124.00 g/mol.
- Say you used one gram more of the sodium carbonate than you did. How would that affect the amount of calcium carbonate you calculate will be formed? How would it affect the amount you actually collect?
- If you used water that already had some sodium carbonate dissolved in it how would that affect the mass of calcium carbonate that you actually collect? How would that compare to the amount of calcium carbonate you calculated would result from the reaction? Explain.
- If you dissolve the sodium carbonate in water that already had some calcium chloride in it how would that affect the mass of calcium carbonate that you actually collect? How would that compare to the amount of calcium carbonate you calculated would result from the reaction? Explain.
- What mass of CaCO3 did you measure in your lab work?
- Is the mass you measured close to the expected mass you calculated based on stoichiometry? Calculate a percent difference between the two. Subtract the calculated value from the measured value and divide the result by the calculated value. Finally, multiply by 100%. If your measured value was smaller, then the percent difference will be negative. If your measure value was bigger, then the percent difference will be positive.
- Would the mass of calcium carbonate that you measure be made larger or smaller if it were not completely dry? Explain.
- Would the mass of calcium carbonate that you measure be made larger or smaller if you did not wash the precipitate before drying it? Explain.
- Using the technique described in the Overview for this lab, calculate the percent yield for your reaction.
- Explain any differences between your measurement and the stoichiometric calculation of the mass of calcium carbonate. In particular, comment on why the percent yield was (likely) not 100%.
- How did this lab help you to learn and apply the skills involved in understanding stoichiometry?
- Pollution is subject to the law of conservation of matter. That is, pollutants do not simply disappear. If a manufacturing plant releases a pollutant into the environment it has to be cleaned up. Usually, a good estimate of the actual amount of dangerous material can be made. Under fortunate circumstances there is a relatively simple chemical reaction which can be used to destroy the polluting chemical. How does stoichiometry help people whose job it is to clean up pollution?